Rare Ophthalmology News
Advertisement
Spotlight On
Tuberous sclerosis complex
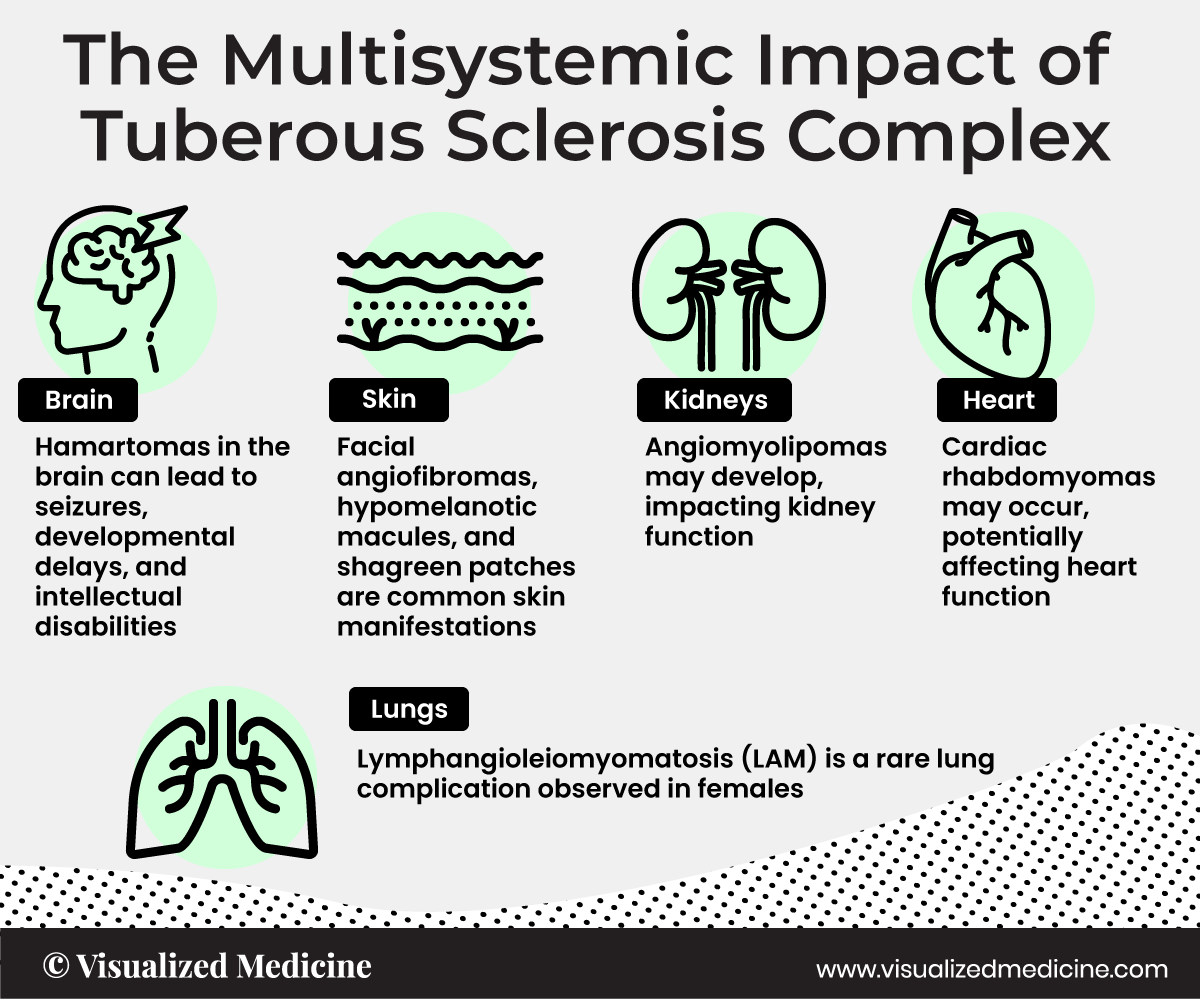
Tuberous sclerosis complex (TSC) is characterized by the growth of benign tumors throughout the body, including in the heart, brain, and kidneys
Rare View
Tuberous Sclerosis Complex (TSC) is a rare genetic disorder characterized by the formation of benign tumors in various organs throughout the body. Neurologically, TSC commonly manifests with the development of cortical tubers, subependymal nodules, and subependymal giant cell astrocytomas in the brain. TSC can also impact other organs such as the skin, kidneys, heart, and lungs, resulting in a multisystemic disorder that requires comprehensive medical management.
Research has shown that early diagnosis and intervention are crucial for optimizing outcomes, particularly in managing neurological manifestations. Current treatment options include antiseizure medications, surgical resection of tumors, and mTOR inhibitors to mitigate tumor growth.
References:
Northrup, H., & Krueger, D. A. (2013). Tuberous sclerosis complex diagnostic criteria update: Recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatric Neurology, 49(4), 243–254. doi:10.1016/j.pediatrneurol.2013.08.001. Krueger, D. A., Northrup, H., & International Tuberous Sclerosis Complex Consensus Group. (2013). Tuberous sclerosis complex surveillance and management: Recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatric Neurology, 49(4), 255–265. doi:10.1016/j.pediatrneurol.2013.08.002.
5 Facts you should know
FACT
TSC causes a combination of symptoms including seizures, intellectual disability, developmental delay, behavioral problems, skin abnormalities, lung disease, and kidney disease.
FACT
The physical manifestations of TSC are due to the formation of hamartia, hamartomas (benign growths such as facial angiofibroma and subependymal nodules), and very rarely, cancerous hamartoblastomas.
FACT
Three types of brain tumors are associated with TSC: giant cell astrocytomas, cortical tubers, and subependymal nodules.
FACT
About 90% of people with TSC develop a range of neurodevelopmental, behavioral, psychiatric, and psychosocial difficulties.
FACT
Around 80% of children under 2 years old with TSC have at least one cardiac rhabdomyoma, and about 90% of those will have several.
Interest over time
Google searches
Common signs & symptoms
Small bumps made up of blood vessels (angiofibromas)
Patches of thickened, rough skin (shagreen patches)
Growths under the fingernails and toenails (ungual fibromas)
Light-colored skin patches (hypomelanonic macules)
Benign brain tumor (astrocytoma)
Abnormal organization of the brain (cortical dysplasia)
Nodules in the brain (subependymal nodules)
Seizures
Current treatments
Everolimus (Brand name: Afinitor)
Manufactured by Novartis Pharmaceuticals Corporation
FDA-approved indication: In April 2018, everolimus (Afinitor) was approved for the adjunctive treatment of adult and pediatric patients age 2 years and older with tuberous sclerosis complex (TSC)–associated partial-onset seizures. In April 2012, it was approved for the treatment of adults with renal angiomyolipoma and TSC not requiring immediate surgery. In October 2010, it was approved for the treatment of patients with subependymal giant cell astrocytoma (SEGA) associated with tuberous sclerosis who require therapeutic intervention but are not candidates for curative surgical resection.
Vigabatrin (Brand name: Sabril)
Manufactured by Lundbeck
FDA-approved indication: For infantile spasms (IS) 1 month to 2 years of age.
Epidiolex (cannabidiol)
Epidiolex (cannabidiol) (CBD) oral solution is FDA approved for the treatment of seizures associated with tuberous sclerosis complex (TSC) in patients 1 year of age and older. Epidiolex was previously approved for the treatment of seizures associated with two rare and severe forms of epilepsy, Lennox-Gastaut syndrome (LGS) and Dravet syndrome (DS). This is the only FDA-approved drug that contains a purified drug substance derived from cannabis. It is also the second FDA approval of a drug for the treatment of seizures associated with TSC.
CBD is a chemical component of the Cannabis sativa plant. However, CBD does not cause intoxication or euphoria (the “high”) that comes from tetrahydrocannabinol (THC). It is THC (and not CBD) that is the primary psychoactive component of cannabis.
Top Clinical Trials
| Title | Description | Phases | Status | Interventions | More Information |
|---|---|---|---|---|---|
| Assessment of Adjunctive Cannabidiol Oral Solution (GWP42003-P) in Children With Tuberous Sclerosis Complex (TSC), Dravet Syndrome (DS), or Lennox-Gastaut Syndrome (LGS) Who Experience Inadequately-controlled Seizures | This study will be conducted to evaluate the safety, pharmacokinetics (PK), and efficacy of adjunctive GWP42003-P in participants \< 2 years of age with tuberous sclerosis complex (TSC), Lennox-Gastaut syndrome (LGS), or Dravet syndrome (DS). | PHASE 3 | RECRUITING | DRUG: GWP42003-P | More Info |
| Adjunctive GNX Treatment Compared With Placebo in Children and Adults With TSC-related Epilepsy | This is a Phase 3, global, double-blind, randomized, placebo-controlled study of adjunctive GNX treatment in children and adults with TSC-related epilepsy. The study consists of a 4-week prospective Baseline phase, defined as the first 28 days following screening, followed by a double-blind phase consisting of a 4-week titration period (Day 1 to Day 28) and a 12-week maintenance period (Day 29 to Week 16). | PHASE 3 | RECRUITING | DRUG: Ganaxalone | More Info |
| Basimglurant in Children, Adolescents, and Young Adults With TSC | The study intends to show that basimglurant provides effective seizure control in children, adolescents and young adults with Tuberous Sclerosis Complex (TSC). | PHASE 2 | RECRUITING | DRUG: Basimglurant | More Info |
| Open-label, Long-term Safety Study of LP352 in Subjects With Developmental and Epileptic Encephalopathy | The objective of this study is to assess the long-term safety, tolerability, and efficacy of adjunctive therapy of LP352 in subjects with developmental and epileptic encephalopathies who completed participation in Study LP352-201. | PHASE 2 | RECRUITING | DRUG: LP352 | More Info |
| Phase 2 Basket Trial of Nab-sirolimus in Patients With Malignant Solid Tumors With Pathogenic Alterations in TSC1/TSC2 Genes (PRECISION 1) | A Phase 2 multi-center open-label basket trial of nab-sirolimus for adult and adolescent patients with malignant solid tumors harboring pathogenic inactivating alterations in TSC1 or TSC2 genes | PHASE 2 | RECRUITING | DRUG: nab-sirolimus | More Info |
Top Treatments in Research
| Agent | Class/Mechanism of Action | Development Status | Company | Clinical Studies | More Information |
|---|---|---|---|---|---|
| DRUG: GWP42003-P | As is the case for many other AEDs, the exact MOA by which CBD produces its anticonvulsant effects is unknown. Cannabidiol is a structurally novel anti-convulsant. Cannabidiol does not exert its anti-convulsant effects through CB1 receptors, nor through voltage-gated sodium channels. CBD may exert a cumulative anti-convulsant effect, modulating a number of endogenous systems including, but not limited to neuronal inhibition (synaptic and extrasynaptic GABA channels), modulation of intracellular calcium (TRPV, VDAC, GPR55), and possible anti-inflammatory effects (adenosine). CBD does not directly bind to, nor activate, CB1 and CB2 receptors at concentrations pharmacologically relevant to its anticonvulsant effect. Among the likely mechanisms of action, modulation of intra-cellular calcium via GPR-55, TRPV, and VDAC is under active investigation in our research laboratories. Additional mechanisms under exploration by our researchers include adenosine modulation, glycine and GABAergic modulation, and serotonin agonism. | PHASE 3 | Jazz Pharmaceuticals | More Info | More Info |
| DRUG: Ganaxalone | Ganaxolone is a GABAA receptor modulator that acts by regulating brain activity. This process can include inhibiting the abnormal electrical discharges that cause seizures and status epilepticus or restoring balance in disrupted neuronal activity in other CNS disorders. | PHASE 3 | Marinus Pharmaceuticals | More Info | More Info |
| DRUG: Basimglurant | Basimglurant (NOE-101) is a highly selective, potent, and cell-penetrant negative allosteric modulator of mGlu5 receptors for the treatment of seizures associated with Tuberous Sclerosis Complex (TSC). mGluR5 blockade has been shown to be effective in controlling seizures in a transgenic animal model of TSC. This effect is explained through a dual mechanism of action: Reducing overactive glutamatergic signaling, an underlying pathology of seizures in TSC Normalizing protein synthesis in the brain, a key feature of TSC | PHASE 2 | Noema Pharma AG | More Info | More Info |
| DRUG: Bexicaserin | Bexicaserin (LP352) is an oral, centrally acting, 5-HT2C superagonist in development for the potential treatment of seizures associated with DEEs such as Dravet syndrome, Lennox-Gastaut syndrome (LGS), tuberous sclerosis complex (TSC), CDKL5 deficiency disorder (CDD), and other epileptic disorders. Bexicaserin is designed to modulate GABA and, as a result, suppress the central hyperexcitability that is characteristic of seizures. | PHASE 2 | Longboard Pharmaceuticals | More Info | More Info |
| DRUG: nab-sirolimus | Exploratory analysis of data in our AMPECT trial suggest responses to nab-sirolimus may occur in patients with TSC1 or TSC2 alterations. Data was generated in non-PEComa patients with TSC1 or TSC2 alterations in the ongoing Expanded Access Program (ASCO 2021 Abstract #339405). In preclinical animal models, nab technology demonstrates greater mTOR pathway inhibition compared to currently approved mTOR inhibitors. | PHASE 2 | Aadi Bioscience, Inc. | More Info | More Info |
Caregiver Corner
Access the latest tools and resources for patients and their caregivers living with TSC

TSC and seizure disorder treatment centers
Hover over the pins to see each center. Click on the location to navigate to their website.
Please note that this is not an exhaustive list and there may be other treatment centers in the United States that are not included. It is also important to consult with a medical professional to determine the best treatment plan for each individual case.
Tools and resources

Creating a future where everyone affected by tuberous sclerosis complex can live their fullest lives
Introductory Booklet to TSC - This booklet contains educational materials about TSC including programs offered by the TSC Alliance, where to turn for additional help, and information on other useful resources. Booklets are free to TSC families and caregivers.
Navigation Guides - Essential information to help guide individuals and families through the complexities of TSC.
- Navigating Transition Years of TSC
- Navigating School Age Years of TSC
- Navigating Early Years of TSC
- Navigating Adult Years of TSC
A Place of Hope Brochure - Learn about the TSC Alliance.
Real Stories From Real Families - Discover the stories of real people who live with Lennox-Gastaut syndrome (LGS), Dravet syndrome, and tuberous sclerosis complex (TSC).
The Care Giver Series - Join actor Greg Grunberg as he travels around the country, sharing the stories of caregivers of families living with Lennox-Gastaut syndrome (LGS), Dravet syndrome, and tuberous sclerosis complex (TSC), and giving them a much-needed day of care.
The TSC Patient Experience:
Learn from more than 950 people living with TSC

Get years of knowledge and experience condensed into a simple report.
Oops! We could not locate your form.
Other resources
Click the drop-down to see info on Patient Advocacy Groups and a Glossary of common terms